
Finding
Hope
HELPING FAMILIES AROUND THE WORLD
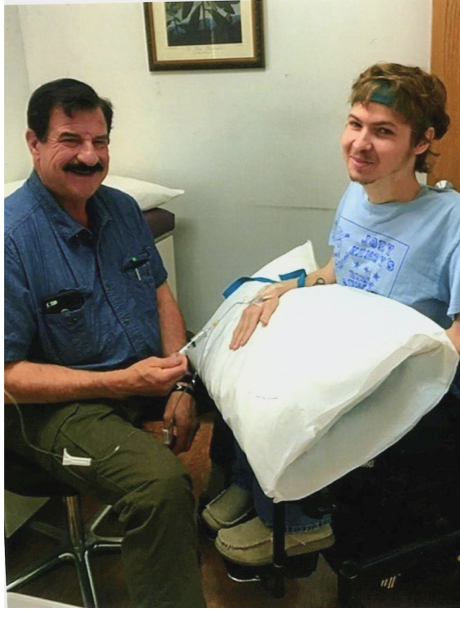
Ryan Benton was diagnosed with Duchenne Muscular Dystrophy, (DMD) at the age of three. DMD is a terminal muscle wasting disease, Doctors told his parents there was no cure and he would be lucky to live past his teenage years. When he was 22 years old, he was offered a chance to be involved in a research study using adult stem cells. This was an unproven method of treatment but Ryan was at a very critical stage in fighting his disease, and had nothing to lose as his time was dwindling. Because Ryan’s parents were childhood friends with Dr. Neil Riordan, who is a world renowned stem cell researcher, they put their faith in Dr. Riordan that this was an opportunity we could not pass up.

Following Ryan’s first round of treatments, he realized he was able to grow muscle for the first time ever, proving to reverse the progression of his otherwise fatal disease. Ryan had a muscle biopsy to confirm our suspicions and the results verified that he was producing dystrophin in the “normal range”. Making Ryan the first person in the world with DMD to be successfully treated with mesenchymal stem cell therapy. At the age of 36, after more than 19 treatments, Ryan felt like he was in much better health than he would have been without the adult stem cell therapy. Unfortunately, Ryan passed away at the young age of 37.
Because of FDA regulations which previously did not permit the use of this form of therapy in the U.S. Ryan received the majority of his treatments at the Stem Cell Institute in Panama City, Panama. In 2015, after six years of traveling out of the country multiple times a year, each time sustaining significant improvements to his overall health, Ryan and Dr. Riordan achieved a huge breakthrough.
There is no limit to how many people ryan’s story can continue to inspire
In 2015, Ryan Benton became the first and only patient with Duchenne Muscular Dystrophy to be granted FDA approval for allogeneic adult stem cell therapy in the United States. An investigational new drug (IND) for compassionate use application was approved, which allowed Ryan to receive treatment in his hometown, Wichita, KS. This was the first instance where an umbilical cord MSCs was approved in the US for treating humans. Approval from the FDA came with many stipulations; however, this form of treatment was to be used for only a single patient, twice a year for 3 years.
In January 2016, the FDA granted an additional treatment per year, which allowed Ryan three total treatments per year, as well as approval for a second compassionate use IND for another patient. This second patient was a six-year-old boy who had also shown success from previous treatments in Panama.
It is a very challenging process to gain this special exemption and unfortunately, it is unlikely this same situation will be duplicated with ease, especially without prior experience in receiving therapy. Our goal now is to use Ryan’s success to help bolster the possibility for other patients with DMD to gain access to therapy in the U.S. This process will likely take another year or more but we are actively taking the steps to advance this likelihood.

Ryan’s Personal Life
While Ryan was passionate about advocating for stem cells, it is important to note that he was much more than a patient with Duchenne’s Muscular Dystrophy. He was also a brother, son, uncle, friend, writer, and a musician. When Ryan was 18 he started the band, The Sunshine Dreamers. Ryan always had a love for making music with his friends, and thanks to stem cell therapy he was able to continue his passion. In 2021 Ryan and his band, The Sunshine Dreamers completed their 9th album.
How Stem Cell Therapy Has Benefited Ryan
Following Ryan’s first treatment, he noticed a renewed strength that he had never felt before. Immediately following each round of treatment, we saw dramatic increases in his overall health, stamina, physical strength, and ease in ability to breathe. Ryan also saw vast improvements in muscle mass and lung capacity as a result of his treatments. Over the course of his treatments, he never experienced any negative adverse side effects.

“In Ryan’s case, they (MSCs) secrete molecules that stimulate his muscles to grow,” said Dr. Riordan. “He doesn’t have the right molecules to stimulate his muscles to grow. These cells have the most potent regenerative capacity of any tool in our tool box right now.”
Maintaining Therapy
Unfortunately, we have found that on average, three to four months after each treatment, the effectiveness of the cells begins to decrease. We believe additional treatments on a consistent regimen of therapy every 3-4 months would help ensure maximum potential when it comes to reversing the progression of this disease. During the FDA 2015-2018 clinical trial (where Ryan received treatments every four months) we observed that his improvements were sustained for longer periods of time and the primary factor to attribute to this is that there was less time between each round of therapy.
Join Us
CTFAC is expanding and we are actively looking for people like you to join our board of directors team. Discover how you can support our strategic growth.
